Abstract
CPX-351 (United States: Vyxeos®; European Union/United Kingdom: Vyxeos® liposomal), a dual-drug liposomal encapsulation of daunorubicin and cytarabine in a synergistic 1:5 molar ratio, is approved for newly diagnosed, therapy-related acute myeloid leukemia (AML) or AML with myelodysplasia-related changes in patients aged ≥1 year in the United States and in adults in the European Union/United Kingdom. In a phase 3 study in older adults with newly diagnosed, high-risk/secondary AML, CPX-351 significantly improved overall survival and remission rates versus conventional 7+3 chemotherapy, with a comparable safety profile. Preclinical data suggest CPX-351 may have synergistic activity with targeted agents, including the FLT3 inhibitor midostaurin (MID).
V-FAST (Vyxeos - First Phase Assessment with Targeted Agents) is an open-label, multicenter, multi-arm, nonrandomized, phase 1b master trial (NCT04075747) to evaluate the safety and preliminary efficacy of CPX-351 combined with targeted agents (MID, venetoclax, enasidenib). Eligible adults in the CPX-351 + MID cohort were aged 18 to 75 years, had newly diagnosed AML (de novo AML or secondary AML) with a FLT3 mutation, were fit for intensive chemotherapy, and had an ECOG performance status of 0 to 2. The dose-exploration phase (3+3 design) determined a recommended phase 2 dose of CPX-351 100 units/m2 (daunorubicin 44 mg/m2 + cytarabine 100 mg/m2) on Days 1, 3, and 5 + MID 50 mg twice daily on Days 8 to 21. There were no dose-limiting toxicities, and additional patients were enrolled in the expansion phase at this dose.
Herein, we report outcomes for a subgroup analysis based on the presence of a FLT3 internal tandem duplication (ITD) and/or tyrosine kinase domain (TKD) mutation within the cohort of adults primarily with de novo AML who were treated with CPX-351 + MID in the V-FAST trial. A total of 23 patients had sufficient data available to be included in the general safety and efficacy analyses in this ongoing trial at the time of the data cutoff on March 22, 2022. Of the patients included in this analysis, 18 patients had a FLT3 ITD mutation and 6 patients had a FLT3 TKD mutation; 1 patient had both FLT3 mutation types and was included in both subgroups. Patient baseline characteristics are shown in the Table. A diagnosis of de novo AML was reported for 78% of patients with FLT3 ITD mutation and all (100%) patients with a FLT3 TKD mutation.
Treatment-emergent adverse events (TEAE) occurring in >50% of patients with a FLT3 ITD or TKD mutation, respectively, included febrile neutropenia (78%; 83%), nausea (67%; 67%), alanine aminotransferase increased (61%; 33%), leukopenia (61%; 50%), and thrombocytopenia (56%; 67%). All patients experienced a ≥3 grade TEAE, primarily hematologic events and infections. Serious TEAEs were reported for 6 (33%) patients with a FLT3 ITD mutation and 2 (33%) patients with a FLT3 TKD mutation; no serious TEAE was reported more than once. Four (22%) patients with a FLT3 ITD mutation and 2 (33%) patients with a FLT3 TKD mutation died during the study; there were no deaths on or before study Day 60.
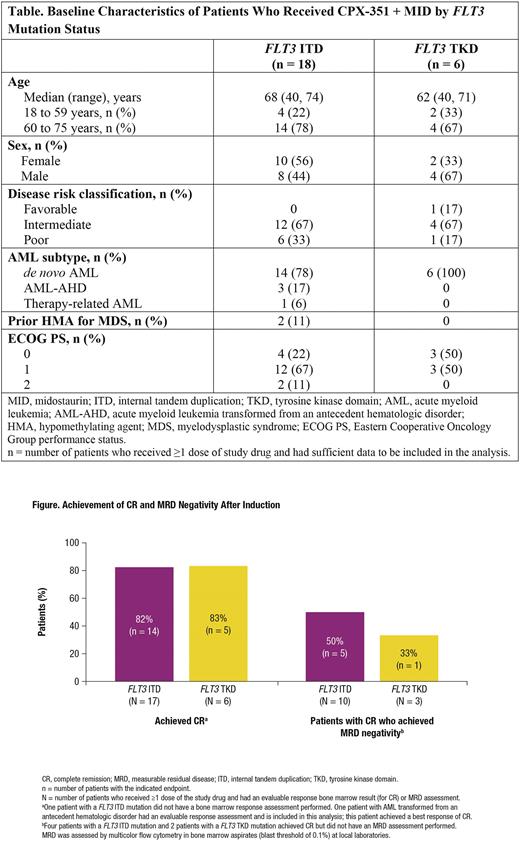
Complete remission (CR) was achieved by 14/17 (82%) patients with a FLT3 ITD mutation and by 5/6 (83%) patients with a FLT3 TKD mutation (Figure). Among patients in CR and with a measurable residual disease (MRD) assessment by multicolor flow cytometry, MRD negativity was achieved by 5/10 (50%) patients with a FLT3 ITD mutation and 1/3 (33%) patients with a FLT3 TKD mutation, all after the first induction cycle. Median (interquartile range) recovery times to an absolute neutrophil count ≥500/μL and platelet count ≥50,000/μL, respectively, were 35 (34, 43) and 34 (28, 36) days for patients in CR with a FLT3 ITD mutation and 30 (29, 35) and 30 (29, 40) days for those in CR with a FLT3 TKD mutation. A total of 10/18 (56%) patients with a FLT3 ITD mutation and 2/6 (33%) patients with a FLT3 TKD mutation have proceeded to hematopoietic cell transplantation to date.
In conclusion, preliminary results from the V-FAST trial suggest the combination of CPX-351 + MID is feasible with a manageable safety profile and promising remission rates in adults with newly diagnosed, FLT3-mutated AML. Although conclusions are limited by the small numbers of patients in each subgroup, results are generally consistent for patients with FLT3 ITD and TKD mutations.
Disclosures
McCloskey:AbbVie, CTI BioPharma, and Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie, Amgen, Bristol Myers Squibb, Incyte, Jazz Pharmaceuticals, Stemline, and Takeda: Speakers Bureau. Pullarkat:AbbVie, Amgen, Genentech, Jazz Pharmaceuticals, Novartis, Pfizer, and Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen, Dova, and Novartis: Consultancy, Other: Advisory Board Member. Mannis:Agios: Consultancy; Astellas: Consultancy; BMS/Celgene: Consultancy; Genentech: Consultancy; Macrogenics: Consultancy; Pfizer: Consultancy; Stemline: Consultancy; Glycomimetics: Research Funding; Astex: Research Funding; Jazz: Research Funding; Forty Seven: Research Funding; Gilead: Research Funding; ImmuneOnc: Research Funding; Syndax: Research Funding; Servier: Consultancy; Abbvie: Consultancy. Lin:AbbVie, Aptevo, Astellas Pharma, Bio-Path Holdings, Celgene, Celyad, Genentech-Roche, Gilead Sciences, Incyte, Jazz Pharmaceuticals, Mateon Therapeutics, Ono Pharmaceutical, Pfizer, Prescient Therapeutics, Seattle Genetics, Tolero, Trovagene: Research Funding. Strickland:AbbVie, BerGenBio, Genentech, Kura Oncology, and Syros: Consultancy, Honoraria. Fathi:MorphoSys, Novartis, Pfizer, Seattle Genetics, Takeda, Trillium Therapeutics, and Trovagene: Consultancy, Membership on an entity's Board of Directors or advisory committees; PureTech: Consultancy; AbbVie, Agios, Amgen, Astellas Pharma, Blueprint Medicines, Bristol Myers Squibb, Daiichi Sankyo, Foghorn Therapeutics, Forty Seven, Inc., Genentech, Ipsen, Kite Pharma, Kura Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Consultancy, Other: Clinical Trial Support; AbbVie, Agios, Bristol Myers Squibb, Servier, and Takeda: Research Funding; Forma: Consultancy; Mablytics: Consultancy; Morphosys: Consultancy; Foghorn: Consultancy; Immunogen: Consultancy; Orum: Consultancy; Kite: Consultancy; EnClear: Consultancy; Amgen: Consultancy; Novartis: Consultancy; Astellas: Consultancy; Abbvie/Servier: Consultancy, Other: Clinical Trial Support; Genentech: Consultancy; Ipsen: Consultancy; Takeda: Consultancy. Faderl:Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Chakravarthy:Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Lutska:Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Chandrasekaran:Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Cheung:Jazz Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Levis:Astellas, and FujiFilm: Research Funding; AbbVie, Amgen, Astellas, Bristol Myers Squibb, Daiichi-Sankyo, FujiFilm, Jazz Pharmaceuticals, and Menarini: Consultancy.
OffLabel Disclosure:
The combination of CPX-351 and midostaurin is considered investigational.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal